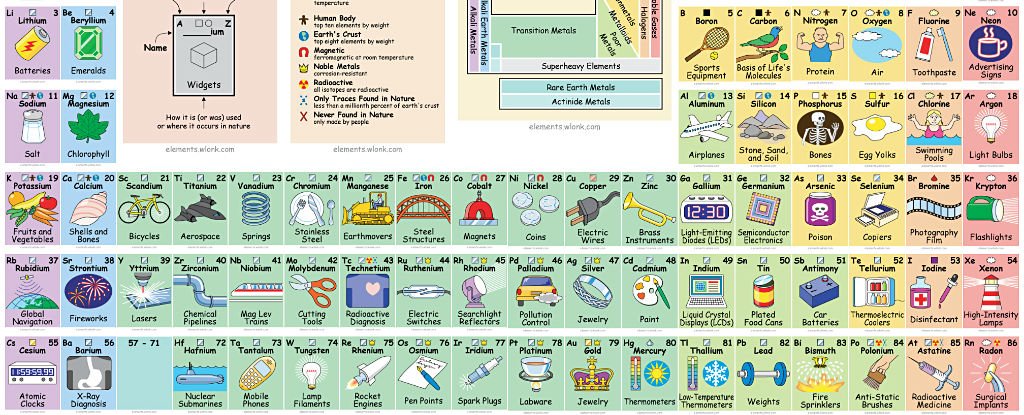
Image showing periodicity of valence s-orbital radius for the chemical elements as size-coded balls on a periodic table grid. The R max values for neutral gaseous element valence orbitals are abstracted from reference 1. Mann, Atomic Structure Calculations II.Hartree-Fock wave functions and radial expectation values: hydrogen to lawrencium, LA-3691, Los Alamos Scientific. Element Vanadium (V), Group 5, Atomic Number 23, d-block, Mass 50.942. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Atomic Number of Vanadium Vanadium is a chemical element with atomic number 23 which means there are 23 protons and 23 electrons in the atomic structure. The chemical symbol for Vanadium is V. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Isotopes of Vanadium. Vanadium has only one stable isotope, 51 V and one radioactive isotope, 50 V, that has a half-life of 1.5×10 17 years. Twenty-four artificial radioisotopes also have been discovered having atomic mass number ranging from 40 to 65. Most stable in these isotopes is 49 V, that has half-life of 330 days.


The Element Vanadium
[Click for Isotope Data]
Atomic Number: 23
Atomic Weight: 50.9415
Melting Point: 2183 K (1910°C or 3470°F)
Boiling Point: 3680 K (3407°C or 6165°F)
Density: 6.0 grams per cubic centimeter
Phase at Room Temperature: Solid

Element Classification: Metal
Period Number: 4
Group Number: 5
Vanadium Dioxide Atomic Number
Group Name: none
Vanadium Atomic Mass Number
What's in a name? Named for the Scandinavian goddess Vanadis.
Taskade chrome. Say what? Vanadium is pronounced as veh-NAY-dee-em.
History and Uses:
Vanadium was discovered by Andrés Manuel del Rio, a Spanish chemist, in 1801. Rio sent samples of vanadium ore and a letter describing his methods to the Institute de France in Paris, France, for analysis and confirmation. Unfortunately for Rio, his letter was lost in a shipwreck and the Institute only received his samples, which contained a brief note describing how much this new element, which Rio had named erythronium, resembled chromium. Rio withdrew his claim when he received a letter from Paris disputing his discovery. Vanadium was rediscovered by Nils Gabriel Sefstrôm, a Swedish chemist, in 1830 while analyzing samples of iron from a mine in Sweden. Vanadium was isolated by Sir Henry Enfield Roscoe, an English chemist, in 1867 by combining vanadium trichloride (VCl3) with hydrogen gas (H2). Today, vanadium is primarily obtained from the minerals vanadinite (Pb5(VO)3Cl) and carnotite (K2(UO2)2VO4·1-3H2O) by heating crushed ore in the presence of carbon and chlorine to produce vanadium trichloride. The vanadium trichloride is then heated with magnesium in an argon atmosphere.
Vanadium is corrosion resistant and is sometimes used to make special tubes and pipes for the chemical industry. Vanadium also does not easily absorb neutrons and has some applications in the nuclear power industry. A thin layer of vanadium is used to bond titanium to steel.
Nearly 80% of the vanadium produced is used to make ferrovanadium or as an additive to steel. Ferrovanadium is a strong, shock resistant and corrosion resistant alloy of iron containing between 1% and 6% vanadium. Ferrovanadium and vanadium-steel alloys are used to make such things as axles, crankshafts and gears for cars, parts of jet engines, springs and cutting tools. D2 item creator 1.13.
Vanadium pentoxide (V2O5) is perhaps vanadium's most useful compound. It is used as a mordant, a material which permanently fixes dyes to fabrics. Vanadium pentoxide is also used as a catalyst in certain chemical reactions and in the manufacture of ceramics. Vanadium pentoxide can also be mixed with gallium to form superconductive magnets.
Estimated Crustal Abundance: 1.20×102 milligrams per kilogram
Estimated Oceanic Abundance: 2.5×10-3 milligrams per liter
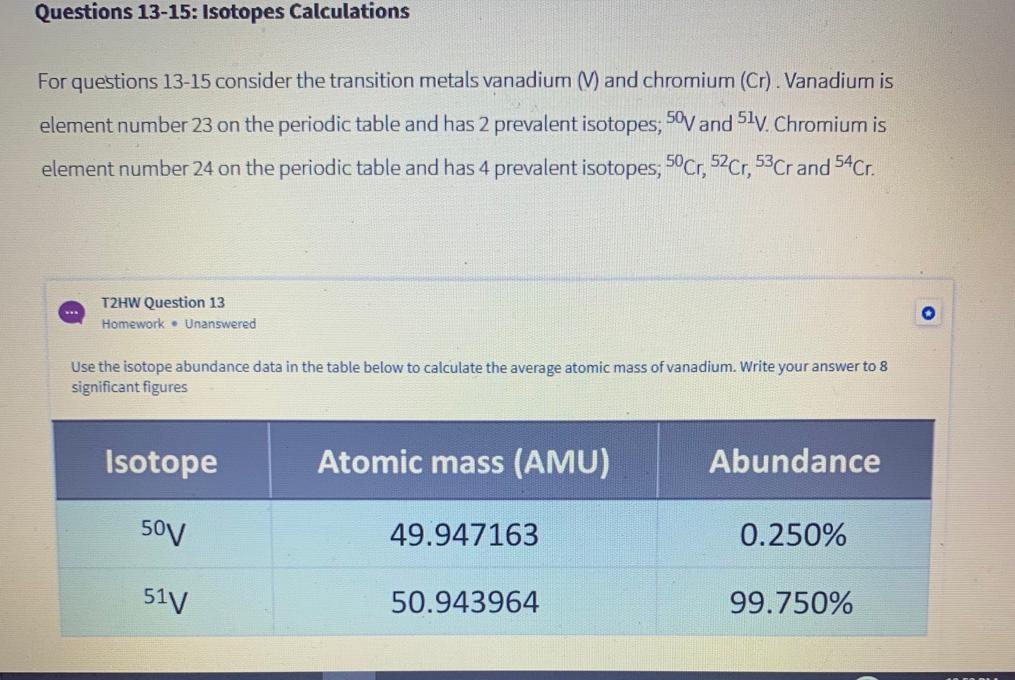
Number of Stable Isotopes: 1 (View all isotope data)
Ionization Energy: 6.746 eV
Oxidation States: +5, +4, +3, +2
Atomic Radius Of Vanadium
Electron Shell Configuration: | 1s2 |
Bitwarden gmail. 2s2 2p6 | |
3s2 3p6 3d3 | |
4s2 |
Vanadium Symbol Element
For questions about this page, please contact Steve Gagnon.
